| 时间:2017-06-15 |
2016年7月27日,国际著名学术期刊《Genome Biology》在线发表了以上海交通大学生物医学工程学院赵小东教授和Bio-X研究院吴际教授为通讯作者的合作课题“Integrative epigenomic analysis reveals unique epigenetic signatures involved in unipotency of mouse female germline stem cells”,论文报道研究人员通过对小鼠雌性生殖干细胞表观遗传修饰谱的研究,发现了决定小鼠雌性生殖干细胞基本生物学特性的表观遗传调控机制。
传统观点认为,女性和绝大多数雌性哺乳动物卵母细胞的产生仅发生在胎儿期,出生后卵母细胞数目不再增加,反而逐年减少,这意味着出生后卵巢无生殖干细胞存在。雌性哺乳动物是否具有生殖干细胞?如果有,是什么分子机制决定其发育单能性和未分化等干细胞特性?
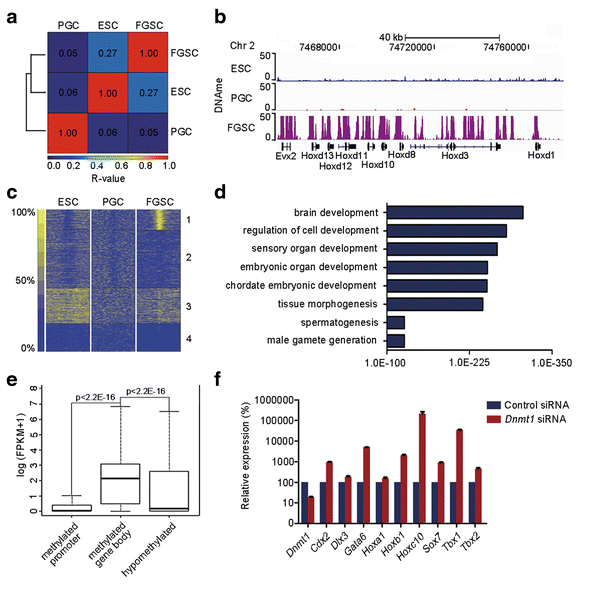
赵小东教授和吴际教授认为,开展小鼠雌性生殖干细胞表观遗传修饰谱研究,他们发现了标记增强子的雌性生殖干细胞特异性的组蛋白修饰标签;更为重要的是,他们的工作揭示DNA甲基化作为一种主要的表观遗传调控机制通过抑制体细胞发育过程来决定雌性生殖干细胞的发育单能性,而且还参与其雌性性别特征的维持;此外,他们还发现生殖细胞相关因子PRMT5对维持雌性生殖干细胞的未分化状态也起到重要作用。
京房生物的专家团队全程参与了这项研究,并承担了重要的角色。京房生物在DNA甲基化领域拥有丰富的经验,有能力提供高质量的MeDIP测序服务。
原文链接:
Integrative epigenomic analysis reveals unique epigenetic signatures involved in unipotency of mouse female germline stem cells
原文摘要:
Background
Germline stem Cells play an essential role in establishing the fertility of an organism. Although extensively characterized, the regulatory mechanisms that govern the fundamental properties of mammalian female germline stem cells remain poorly understood.
Results
We GENErate genome-wide profiles of the histone modifications H3K4me1, H3K27ac, H3K4me3, and H3K27me3, DNA methylation, and RNA polymerase II occupancy and perform transcriptome analysis in mouse female germline stem cells. Comparison of enhancer regions between embryonic stem cells and female germline stem cells identifies the lineage-specific enhancers involved in germline stem cell features. Additionally, our results indicate that DNA methylation primarily contributes to female germline stem cell unipotency by suppressing the somatic program and is potentially involved in maintenance of sexual identity when compared with male germline stem cells. Moreover, we demonstrate down-regulation of Prmt5 triggers differentiation and thus uncover a role for Prmt5 in maintaining the undifferentiated status of female germline stem cells.
Conclusions
The genome-wide epigenetic signatures and the transcription regulators identified here provide an invaluable resource for understanding the fundamental features of mouse female germline stem cells.